The EQIPD Vision
Improve preclinical research by increasing research quality
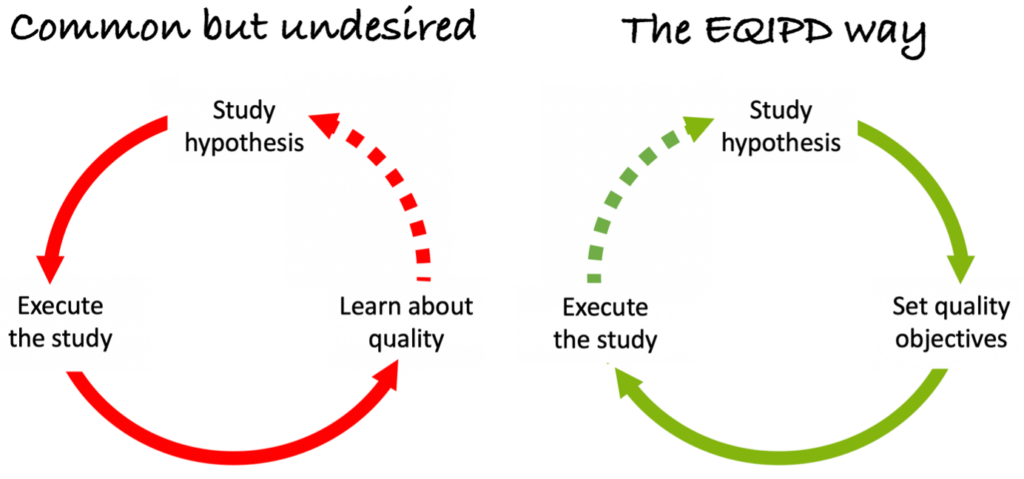
There are no comprehensive, generally agreed and universally applicable guiding principles and criteria governing rigour in the design, conduct and analysis of pre-clinical and safety research, although there is emerging consensus for the standards of reporting such research.
As a result, the development of new medicines has slowed dramatically in the last 10 years, with the number of drugs in development down by 70% since 2005. This is mostly due to the complexity of drug development leading to a significant failure rate.
The EQIPD project, which was active from 2017 to 2021 aimed to reverse this trend.
This consortium that united more than 20 research groups from industry and academia has developed strategies to ensure that early drug development research proceeds along structured lines.

The Guarantors of EQIPD were founded in 2021 with the goal to carry on the these efforts and to disseminate the developed strategies and results with the entire research community.
We believe in the community need for simple, sustainable solutions that facilitate improvements in data quality without impacting innovation and freedom of research.

