GoEQIPD Governance Model
Background
The Guarantors of EQIPD (GoEQIPD) e.V. were founded in 2021 as a non-profit organization to oversee the implementation of the EQIPD Quality System, which was developed in the context of the European Quality in Preclinical Data Innovative Medicines Initiative project which ran from 2017 to 2021 and received more than €4m in public funding.
Quality System
The development of the EQIPD Quality System has been supported by a stakeholder group of over 100, and we have partnered with several organisations including Scientist.com to develop ways of implementing the EQIPD standards within their processes. Three laboratories have received full EQIPD accreditation in the pilot phase. The framework for the EQIPD Quality System is in the public domain and can be used by any who wish to do so.
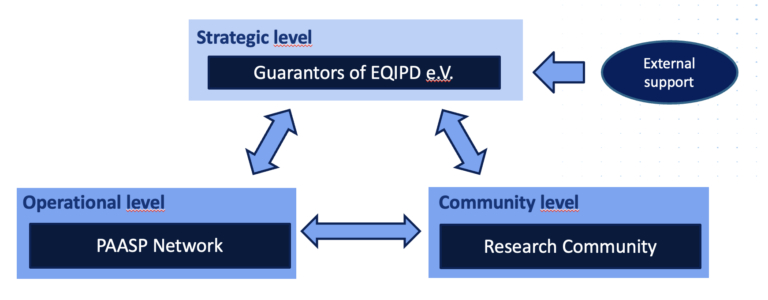
The Guarantors of EQIPD partner with PAASP
In November 2021 GoEQIPD publicly solicited proposals from organisations wishing to help implementing a framework through which they might provide mentoring and accreditation to laboratories wishing to show that they meet EQIPD QS standards, under the direction of GoEQIPD. PAASP GmbH has responded to the call and GoEQIPD is currently working on a contract aiming to establish a partnerships with PAASP as the operational arm of the EQIPD governance. It is expected that the operational arm would provide fee-based services for laboratories wishing to implement the EQIPD quality system and seek EQIPD certification.
EQIPD QS governance
Community events such as the EQIPD summer school that in the past have brought scientists from all over the world together to learn about quality assurance, rigor and the reproducibility will be offered in the future. EQIPD will also collaborate with partners on other educational formats and events to further engage with the preclinical research community.

