Enhancing Quality In Preclinical Data
7
Certified sites
18
Core Requirements
30
consortium Partners
80+
Stakeholder
Our vision
EQIPD is a fit for every member of the research community
Pharma industry
… invests into the most expensive phases of drug R&D.
Clinical studies based on preclinical evidence lacking robustness are likely to fail.
EQIPD provides solutions to improve data integrity.
Private and public funders
… support a great number of projects guided by their scientific excellence.
Research quality assessment is a filter that can be applied to focus investments on most robust and therefore more likely to translate research.
Research organizations
… increasingly depend on successful collaborations and sustainability of research output.
EQIPD offers several possibilities to review, implement and maintain best research practices.

Scientific Excellence vs. Research Quality
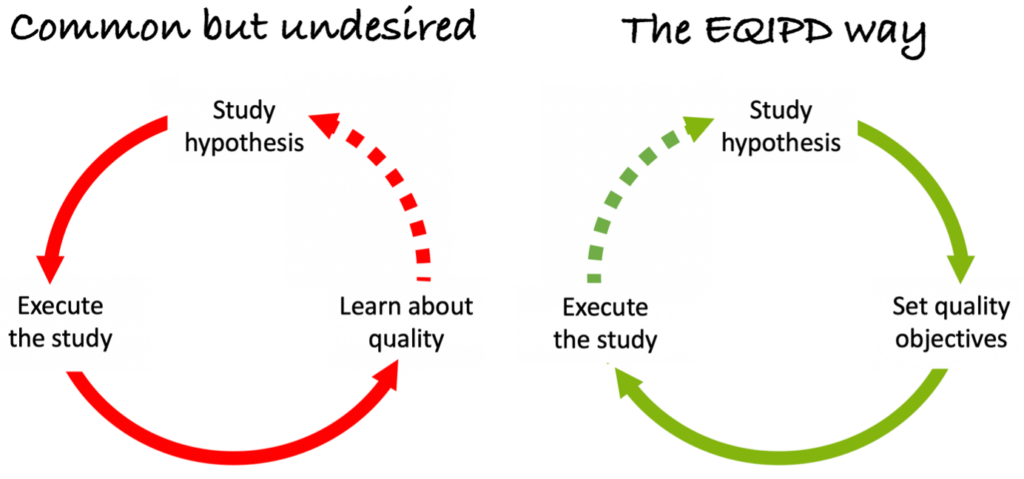
Scientific excellence is the key to advance science but it does not guarantee that the conducted experiments deliver robust results. There are two primary reasons for that. First, education in science does not always focus on the requirements for delivering of robust data. Second, excitement associated with a scientific hypothesis or conveyed by a scientific leader may introduce bias in study design, conduct, analysis and/or reporting.
Regulated vs. Non-regulated
In the drug discovery and development process, there are several steps that have adequate quality control and are covered by GxP policies (e.g. Good Laboratory Practice, GLP).For non-regulated areas (most specifically, biology and pharmacology of drug discovery projects), GLP-like procedures would not be fit for purpose and may not help to secure the quality of research. In fact, one may indeed imagine a lab running under GLP conditions but nevertheless still failing to design and execute robust studies.


Good Research Practice vs. Research Integrity
Responsible conduct of research has multiple facets and the examples of suboptimal or unacceptable practices go well beyond the commonly known definition of research misconduct (fabrication, falisfication or plagiarism).In addition to such direct violations of the good research practices, examples of other unacceptable practices include manipulating authorship, withholding research results, reporting of results so as to introduce or promulgate bias, failing to document essential steps or results of the research process.
EQIPD WEBINAR SERIES 2023
Martien Kas / Piotr Popik
Use cases of the EQIPD quality system
In this webinar Martien and Piotr present their take on the EQIPD quality system and how it was implemented in their research environment. They provide interesting use cases and examples on the implementation process as well as benefits of having such a system.
Find out more about…
the EQIPD QS
the EQIPD project
Sign up for our Newsletter.
Which is published every two months and informs about developments in the field of Good Research Practice.
-
New EQIPD Certification Awarded: Congratulations to Childlight at the University of Edinburgh!
We are delighted to announce that Childlight at the University of Edinburgh has successfully achieved the EQIPD certification. The Childlight – Global Child Safety Institute conducts primary research on the situation of children worldwide and develops influential indices that shed light on the realities children face across different regions and contexts. Their work contributes substantially to evidence based policy…
-
3R Symposium in Oulu
On my way home from an inspiring event that left me with important reflections and wonderful impressions: breathtaking winter landscapes, delicious food, and fantastic colleagues. Pauliina Rautio and Mikko Karpale opened the symposium with two thoughtful and insightful presentations on caring: caring for animals, caring for research, and caring for people. Pauliina also offered a very…
-
iRISE Webinar: the EQIPD Quality System
In this webinar, you’ll learn about the benefits and requirements of the EQIPD Quality System (QS), a framework designed to enhance the reliability and transparency of preclinical research. The session explains how EQIPD QS helps research teams establish clear, efficient, and compliant processes without adding unnecessary bureaucracy. We also highlight its role within the EU-funded…

